Contact project managers :
- Radia Tamarat
- Christelle Huet
- Marie-Odile Bernier
- Sophie Jacob
- Marc Benderitter
The benefits of controlled use of ionizing radiation in medicine are well established. In just a few years, radiotherapy, imaging and radiology technologies have made enormous strides. The MEDIRAD (MEDedIcal low-dose RADiation exposure) project aims to answer a central question: "How can we improve radiation protection for patients and healthcare professionals to ensure that progress benefits everyone, in complete safety? This project aims to improve scientific knowledge of the risks associated with the use of ionizing radiation in medicine, and to optimize its use for diagnostic and therapeutic purposes. The project also aims to promote links between science, medicine and society, for a better quality of life for patients and better protection for healthcare professionals.
Photo: Patient on a Clinac 2100C linear gas pedal treatment table for a radiotherapy session. Location: © Médiathèque IRSN
Completion dates: 2017-2022
Budget: 10 million euros
Partners: 33 partners from 14 European member states
Scientific coordination: ISC Global (Spain)
Medical coordination: Université Paris Descartes (France)
Project coordination: European Institute for Biomedical Imaging Research - EIBIR (Austria)
Ionizing radiation in medicine is used for diagnosis and treatment. Its growing use in medicine raises a number of radiation protection issues for patients and healthcare professionals. In Europe, exposures resulting from CT scans, radiography, fluoroscopy, interventional radiology and nuclear medicine contribute 57%, 17%, 12%, 9% and 5% respectively of the collective dose. Over 45 million CT scans and 1.6 million radiotherapy sessions are delivered every year in Europe, with a steady increase in these medical procedures of around 5% per year.
Progress in medical radiation protection is set to continue, thanks to technological innovation in healthcare, the promotion of best practices established by associations of healthcare professionals, and the personalization of care. These advances are underpinned by advanced scientific knowledge, which is the foundation of the public health challenges to be met if ionizing radiation is to be used safely in medicine.
Multidisciplinary research (expertise and science, science and medicine) on a European scale is needed to build optimized medical radiation protection. Recent advances in biotechnologies and computer technologies ("Big Data" and artificial intelligence) offer unique opportunities.
A strong link between science and society remains essential to guide research priorities in medical radiation protection, to set the appropriate ethical rules for medical research and to accelerate the transition from research results to everyday medical practice; thus enabling every European patient to have equitable access to the most optimized diagnostic and therapeutic protocols.
Coordinated by ISC Global and Paris Descartes University, the MEDIRAD project brings together 33 partners, including INSERM, the International Agency for Research on Cancer (IARC), Paris Descartes University and IRSN for France. The project is a platform for integrating different medical disciplines (nuclear physicians, radiologists, radiotherapists, hospital physicists, cardiologists and oncologists), scientific disciplines (radio-biologists, radio-pathologists, radio-toxicologists, dosimetrists and epidemiologists, as well as scientific experts in cardiac physiology and oncology) and radiation protection expertise, in order to improve the quality of care and the protection of healthcare professionals.
The MEDIRAD project is structured into 6 WPs, 4 of which are dedicated to optimizing radiation protection in interventional radiology (WP2), nuclear medicine (WP3), radiotherapy (WP4) and medical imaging (scans) (WP5). The aim of WP6 is to produce recommendations for medical radiation protection, based on the scientific and technical output of WPs 2-5.
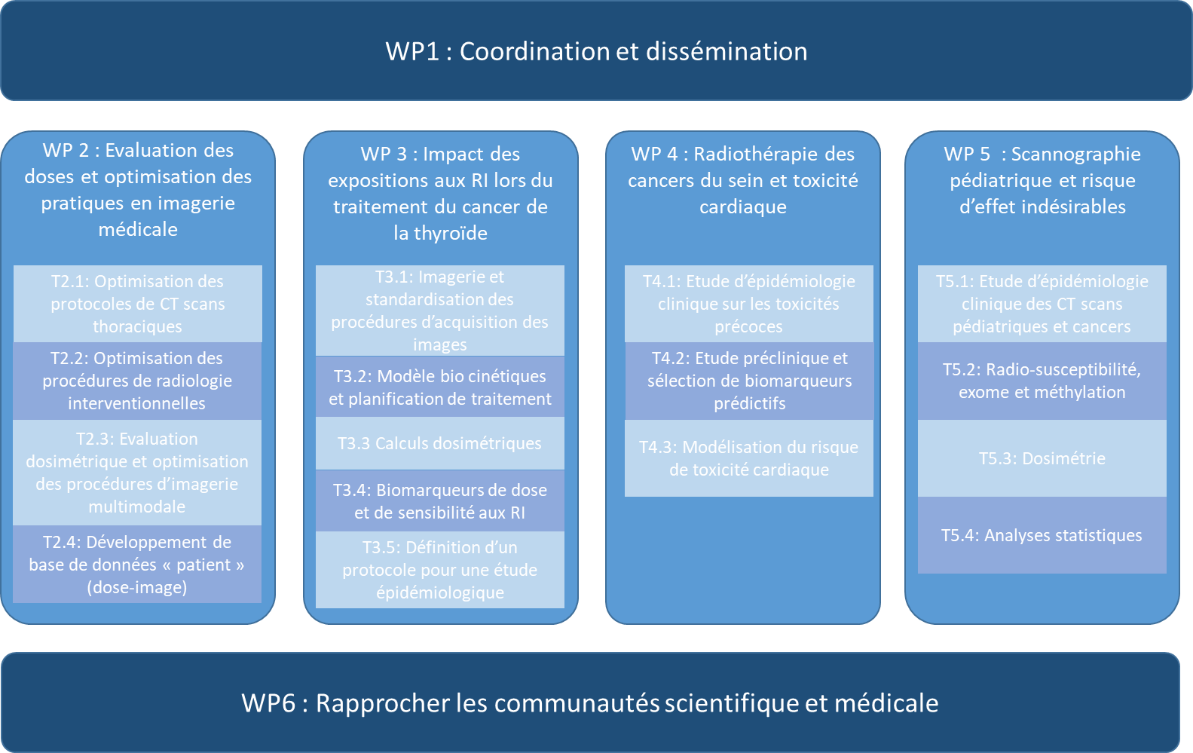
Figure 1: MEDIRAD project structure. IRSN leads WP6, co-leads WP4, leads T4.1 and T4.2, and participates in T2.2, T4.3 and T5.1.
As a major contributor to this project, IRSN has participated in MEDIRAD's work in three areas where radiation is used in medicine: interventional radiology, radiotherapy and medical imaging.
Based on the scientific results produced by MEDIRAD's partners, IRSN coordinated the drafting of a set of radiation protection recommendations with the support of stakeholders, including patient associations, healthcare professionals' associations, radiation protection authorities and manufacturers in the medical sector.
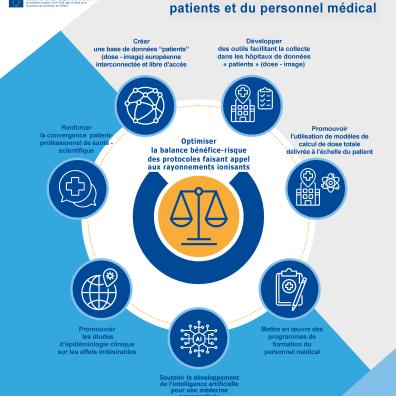
Figure 2: Infographic on MEDIRAD recommendations for strengthening radiation protection for patients and healthcare professionals
These recommendations, available in their complete version on the MEDIRAD website, are grouped into 4 major areas:
These include the importance of developing (i) standardized methods for data coding, (ii) ergonomic user interfaces, (iii) data storage infrastructures and (iv) data management plans for the collection and secure storage of patient data; these "patient" databases (dose - image) should be linked to "patient" bio-banks to maximize their exploitation.
In particular, it is important to assess the organ dose for each medical diagnostic procedure involving CT Scans.
The importance of deploying quantitative imaging in nuclear medicine departments for standardized, individualized use of radiopharmaceuticals in Europe.
The importance of continuing research into the undesirable effects of ionizing radiation on healthy tissue, and the definition of a European research strategy for the development of AI applications in medical radiation protection.
To date, some thirty scientific articles have been published as part of this project.
In interventional radiology, where research has focused on radiation protection for healthcare professionals and optimizing practices. In concrete terms,
IRSN took part, in collaboration with 2 partners (Beligium Nuclear Research Centre-SCK CEN and Nofer Institute of Occupational Medicien - NIOM), in a study of the effectiveness, particularly in terms of the crystalline lens and the brain, of radiological protection used by interventional radiology personnel. Using Monte Carlo simulations and measurements on phantoms and personnel, various types of protection were investigated: visors, caps, aprons, drapes and the Zero-gravity system (suspended leaded suit). Based on this work, it is recommended that the performance of protective equipment be tested under conditions of clinical use. Recommendations have also been drawn up in collaboration with stakeholders, for the attention of decision-makers, authorities and learned societies.
In radiotherapy, where research has focused on assessing the risks of cardiac toxicity associated with breast cancer radiotherapy and optimizing practices. In concrete terms,
In medical imaging, work has focused on assessing the risk of cancer associated with pediatric scans. Two epidemiological studies have investigated the risk of cancer following scans on pediatric cohorts (exposure during childhood or adolescence). These studies, coordinated by ISGlobal, were conducted with 3 other European partners (IRSN, University of Newcastle, NKI).
In concrete terms,
Laboratories: Laboratory for the radiobiology of accidental exposure (LRAcc), Ionizing Radiation Dosimetry Laboratory (LDRI), Ionizing Radiation Epidemiology Laboratory (LEPID)

The laboratory is located in Fontenay-aux-Roses and conducts research in radiobiology, biological dosimetry, radiopathology and regenerative medicine

The main research focus of the Ionizing Radiation Dosimetry Laboratory is dosimetric assessment in the event of external exposure
LEPID conducts research into the effects on human health of occupational, medical or environmental exposure to low doses of ionizing radiation. This work is based on the epidemiological monitoring of cohorts and innovative statistical analyses, in collaboration with partners in France and other countries.
Contact project managers :